AMS is a Powerful Tool for Nuclear Forensics
Nuclear forensics, which can be applied to both interdicted materials and debris from a nuclear explosion, is the application of laboratory analysis and interpretation to provide technical conclusions (provenance, design, etc.) about a nuclear device or interdicted nuclear material.
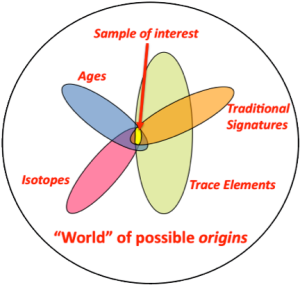
Nuclear forensic analysts can build confidence in their conclusions by employing multiple signatures that collectively minimize the subset of possible matches between known materials and an interdicted sample [Fig 1].
The unique capabilities of AMS are well-suited to complementing the wide range of analytical tools that are applied to nuclear forensics. Because it is insensitive to molecular isobaric interferences, AMS is the preferred technique for measuring ultra-trace concentrations of rare, long-lived radionuclides in the presence of an overwhelming stable isotope. Isotope ratios of 10-12 to 10-15 are routinely measured by AMS and analytical backgrounds as low as 10-17 have been attained for some isotopes. The abundance sensitivity of AMS offers the potential to develop new forensic signatures.
Detecting the presence of trace quantities of anthropogenic uranium in the environment
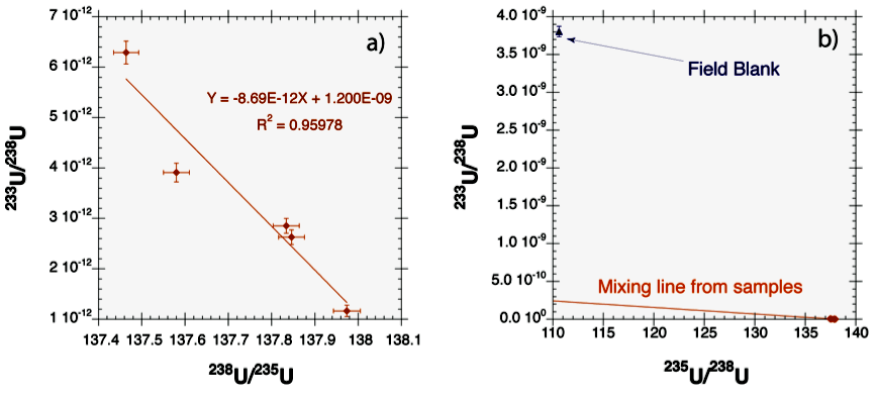
One example of a new forensic signature enabled by AMS is 233U. Uranium-233 is produced in nuclear fuel by neutron-capture on 232Th and by alpha-decay of 237Np. Since there is no appreciable natural background, the presence of 233U can be a sensitive indicator that anthropogenic uranium has been blended with natural uranium. Furthermore, since it has a unique production pathway, 233U can be used to distinguish different sources of anthropogenic uranium. In a previous study [Tumey 2009], samples that were expected to contain only natural uranium were collected from a site with known anthropogenic contamination. The major uranium isotope ratios showed a deviation from natural values indicating the presence of recycled uranium, although it was not clear whether the source was residual contamination from the collection site or anthropogenic uranium intrinsic to the sample. We constructed a multi-isotope mixing plot [Fig. 2] using 233U to reveal that the source of non-natural uranium was not associated with contamination from the site, a finding that was contrary to the expectations given the declared history of these samples.
Attribution of refined uranium materials to parent ore bodies
Naturally-occurring 236U can also be exploited as a nuclear forensic signature. Generally considered to be an anthropogenic isotope of uranium, 236U is also produced at very low levels in nature by neutron capture on 235U. It has been demonstrated that 236U/238U ratios in uranium ores can vary from 10-13 to 10-10 and are influenced by the geologic context of the ore body [Table I]. Consequently, 236U would be a strong complement to the existing isotopic signatures used in UOC attribution. AMS is currently the only technique capable of analyzing samples across the entire natural range of 236U/238U.
| Material Origin | 236U/238U (x 10-11) |
|---|---|
| Beaverlodge, Canada | 28.85 ± 0.95 |
| ESI, USA | 1.128 ± 0.82 |
| Randstadt, Sweden | 512 ± 13 |
| El Mesquite, USA | 2.06 ± 0.12 |
| Kerr McGee, USA | 722.6 ± 9.9 |
| Falls City, USA | 1.698 ± 0.076 |
| Czech Republic | 343.7 ± 3.8 |
Table I. 236U/238U isotope ratios measured in a selection of uranium ore concentrate (UOC)
Inferring the neutron fluence from a nuclear detonation using long-lived activation products
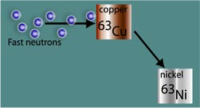
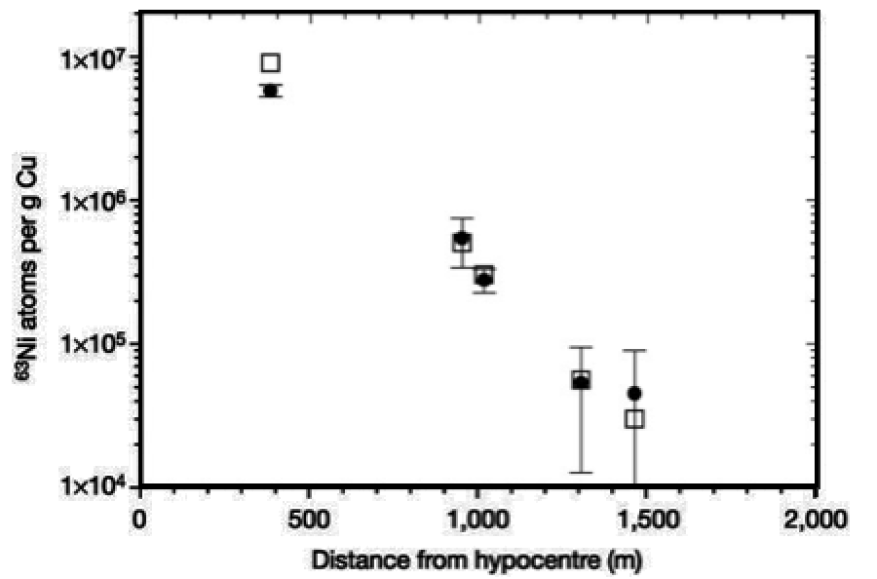
AMS can also play an important role in post-detonation nuclear forensics. The relative abundance of neutron activation products in the debris produced by a nuclear detonation can provide important clues as to the design and the materials used in a nuclear device. This information can be used for source attribution which is essential to deterring nuclear weapon states from providing assistance to terrorist organizations. AMS has been previously employed in the measurement of neutron activation products such as 63Ni in samples of copper to reconstruct the dose associated with the Hiroshima detonation [Figs. 3&4]. In this study, the high abundance sensitivity of AMS was essential to perform the measurements. This feature also makes AMS well-suited to measurement of other trace activation products (e.g., 10Be, 26Al, 41Ca) for nuclear forensics.
Selected References
Tumey SJ, Brown TA, Buchholz BA, Hamilton TF, Hutcheon ID, Williams RW. (2009) Ultra-sensitive measurements of 233U by accelerator mass spectrometry for national security applications. Journal of Radioanalytical and Nuclear Chemistry 282: 721-724.