14C “Bomb Pulse” dating as a Forensics Tool
Traditionally, radiocarbon dating has been considered to be an archeological tool rather than a forensic one.
Radiocarbon or carbon-14 (14C) is produced naturally in the atmosphere by cosmic ray interactions with nitrogen. Single carbon atoms in the atmosphere are quickly oxidized to carbon dioxide CO2. The atmospheric concentration of natural 14C with respect to all carbon has remained relatively stable at about 1.2 parts per trillion over the past several thousand years. With a radioactive half-life of 5730 years, the radioactive decay of 14C is minimal within the time periods of interest in medical forensic cases and applicable for samples over 300 years of age.
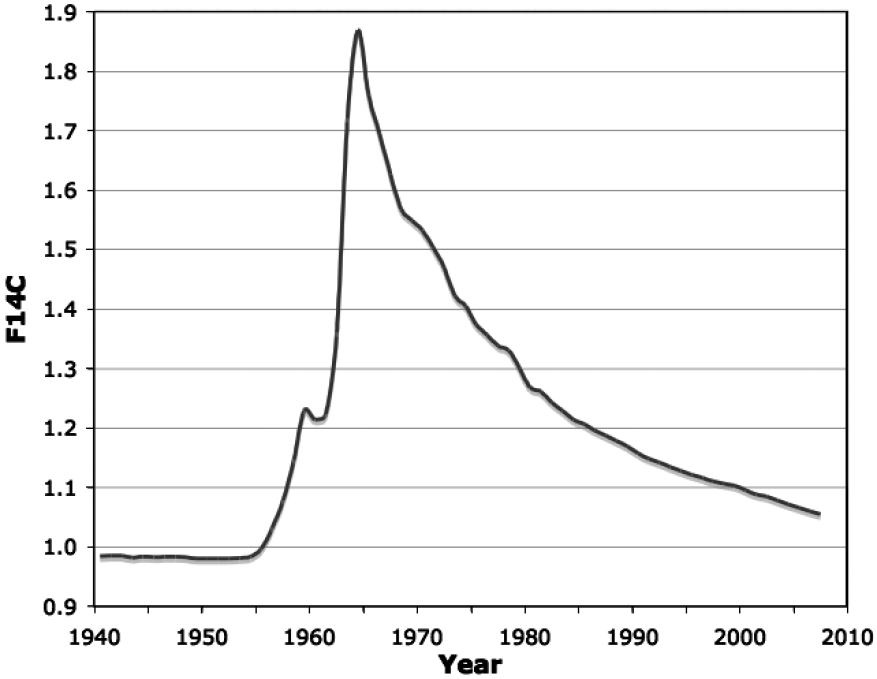
Atmospheric testing of nuclear weapons during the 1950s and early 1960s doubled the concentration of 14C/C in the atmosphere (Figure 1). From the peak in 1963, the level of 14CO2 has decreased with a mean life of about 16 years, not due to radioactive decay, but due to mixing with large marine and terrestrial carbon reservoirs. The 14C has not actually disappeared, it has simply moved out of the atmosphere. The temporal variations of artificially high levels of atmospheric radiocarbon have been captured in organic material world-wide and thus offer an opportunity to determine a date of synthesis for biomolecules. Since radiocarbon is incorporated into all living things, this pulse is an isotopic chronometer of the past half-century.
The atmospheric 14CO2 curve depicted in Figure 1 is a northern hemisphere annual growing season average. The isotopic content of new plant growth reflects the atmospheric radiocarbon concentration. New leaves are produced in weeks while larger fruit and vegetables form over the period of a month or two. The atmospheric concentration of 14C becomes the fingerprint of this radioisotope in a given year's food supply. Herbivores lag the atmosphere slightly because their primary carbon source is on the order of months old. Omnivores and carnivores lag the atmosphere further because their carbon sources are another step removed. However, it has been confirmed that atmospheric 14C levels from the bomb pulse closely correlate with 14C found within dietary components from that given year and that the annual 14C averages in food match those found in human tissue.
Within organisms, tissues turn over at different rates so 14C levels vary between tissues. The date of formation of a tissue or specific biomolecule can be estimated from the bomb-curve by considering these lags in incorporation and relating the 14C concentration with the date. Using an annual average of the carbon intake over a growing season can account for much food chain lag and produce a usable curve (Figure 1). Caution must be exercised when dating an elevated sample since the pulse is double valued. Placing a sample on the ascending or descending side of the pulse can often be accomplished if other information is available.
The precision of bomb-pulse dating depends on the ability to measure the 14C concentration in a sample and the slope of the curve. It is relatively easy to achieve 0.5-0.8% precision when analyzing recent full-sized samples. As the slope of the pulse flattens, the uncertainty in 14C analysis translates into a larger chronological uncertainty. When the slope was steep, the uncertainty was typically ± 1 year. Since 2000, that same measurement precision yields a chronological uncertainty of ± 2-4 years. The technique of 14C bomb pulse dating can be applied to documents, soft human tissues, hair, bone, cartilage, teeth, seeds, pollen and bacteria."
Selected Publications
Sarachine Falso MJ, Buchholz BA. (2013) Bomb Pulse Biology. Nuclear Instruments & Methods in Physics Research, Section B-Beam Interactions with Materials & Atoms. 294:666-670.