A critical scientific and policy oriented question is what are the present day sources and sinks of carbon dioxide (CO2) in the natural environment and how will these sinks evolve under rising CO2 concentrations and expected climate change and ecosystem response.
Sources and sinks of carbon dioxide impart their signature on the distribution, concentration, and isotopic composition of CO2. Spatial and temporal trends (variability) provide information on the net surface (atmosphere to ocean, atmosphere to terrestrial biosphere) fluxes. The need to establish more reliable estimates of sources and sinks of CO2 has lead to an expansion of CO2 measurement programs over the past decade and the development of new methodologies for tracing carbon flows. These methodologies include high-precision pCO2, δ13CO2, and [O2/N2] measurements on atmospheric constituents which when combined have allowed estimates of the net terrestrial and oceanic fluxes at decadal timescales. Major gaps in our understanding remain however, and resulting flux estimates have large errors and are comparatively unconstrained.
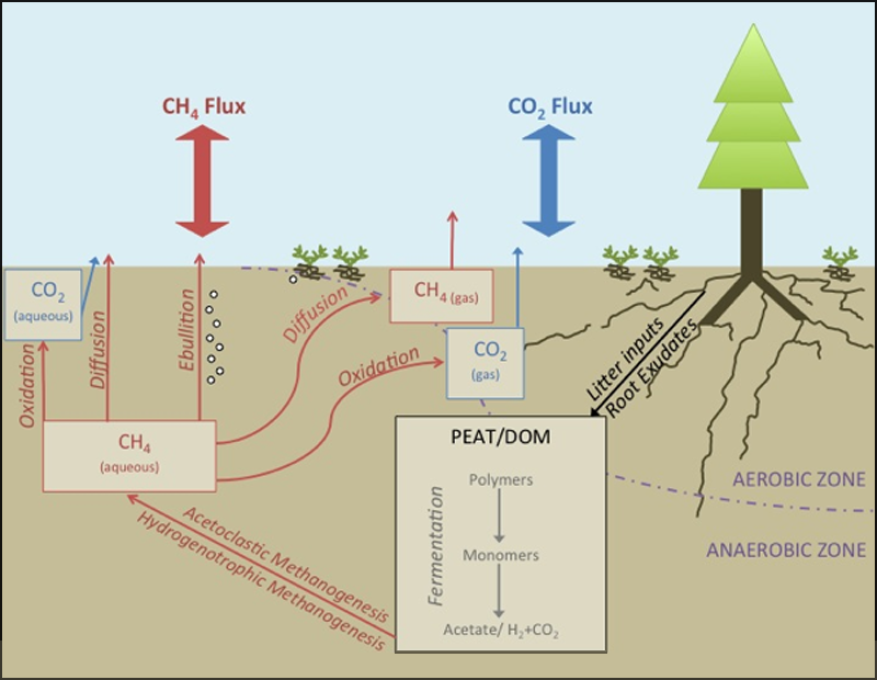
A similar question exists for methane (CH4). Methane (CH4) is a potent greenhouse gas (~70X stronger than CO2) but atmospheric concentrations of CH4 under future climate and land-use change are uncertain in part because sensitive ecosystems release both CH4 and carbon dioxide (CO2) and it is unknown how these systems will partition future releases of carbon to the atmosphere. Ecosystem observations of CH4 emissions lack mechanistic links to the processes that govern CH4 efflux including microbial production, oxidation, upward transport by ebullition, and diffusional transport (Figure 1). Understanding these processes and their interactions is critical for prediction of biospheric feedbacks to climate change.
One potentially powerful approach to tracking carbon flows is based on observations of the 14C/12C ratio of atmospheric CO2 or 14CH4. In the case of CO2, this ratio can be used to explicitly distinguish fossil-fuel CO2 from other sources of CO2 and also provide constraints on the mass and turnover times of carbon in land ecosystems and on exchange rates of CO2 between air and sea.
To tease apart the processes of methane production and consumption and to attribute emissions to belowground sources, one can use the natural abundance isotopic characterization (14C, 13C, 2H) of CH4, CO2, and the physical sources of carbon that provide fingerprints of the biogeochemical pathways that cycle carbon through the landscape. 13C and 2H can be used for separation of methanogenesis (which substantially fractionates against 13C) and methanotrophic methane consumption (in which fractionation of 2H and 13C are correlated to one another). We use 14C measurements of CH4 to identify carbon sources being utilized under different treatments (older peat and subsurface carbon vs younger, recently fixed dissolved organic carbon).
In addition to natural sources of CH4 and those due to land-use change, CH4 is released from a variety of industrial activities. Bottom up and top-down inventories of the release of CH4 do not match with the implication being that estimates of CH4 release during industrial activities are lower than they should be. Direct analysis of CH4 concentrations, isotopic analysis of CH4, and tracer-tracer relations including those of isotopes can be used to elucidate the sources of CH4 and the potential under-estimate of e.g., flaring, or leakage from infrastructure.