The Technique of Accelerator Mass Spectrometry
AMS is a technique for measuring isotope ratios with high selectivity, sensitivity, and precision.
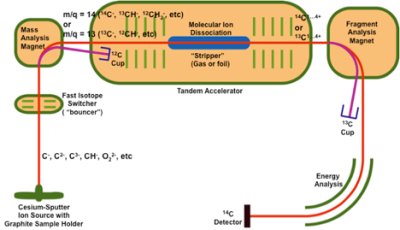
In general, AMS separates a rare radioisotope from stable isotopes and molecular ions of the same mass using a variety of standard nuclear physics techniques. In the case of carbon, 14C ions are separated and counted as particles relative to 13C or 12C that are measured as an electrical current. The key steps of AMS allowing quantitative and specific measurement of isotopes are the production of negative ions from the sample to be analyzed, a molecular disassociation step to convert the negatively charged molecular ions to positively charged nuclei, and the use of high energies (MeV) which allow for the identification of ions with high selectivity.
14C -AMS analysis requires samples be converted to a form that retains the isotopic ratio from the original sample and that provides chemical and physical equivalence for all carbon atoms. Historically, samples for 14C -AMS analysis have been first combusted to produce CO2, followed by a catalytic reduction to form graphite. These samples are bombarded by a Cs+ beam forming negative elemental or molecular ions. The production of negative ions removes the primary isobaric interference for radiocarbon, 14N, because nitrogen does not form a stable negative ion. Ions are subsequently selected at single atomic mass units through a low energy magnetic analyzer by switching the electrostatic potential on the magnets vacuum chamber. 12C is separated from mass 13 and 14 ions and can be quantified in a Faraday cup. This low energy mass spectrometer cannot resolve the small differences among the rare isotope and the nuclear and molecular isobars. Hence negative ions and molecules are accelerated to at least a few hundred keV energies in the first stage of a tandem accelerator (the schematic diagram below shows our 1 MV tandem accelerator AMS system). At the end of this first acceleration stage, these ions pass through a molecular dissociator, or, stripper. Molecular ions, such as 13CH are dissociated in collisions with the atoms in the stripper that also removes one or more electrons from the residual atomic ions. The 14C + ions are further accelerated followed by momentum and energy analysis before being counted using standard particle detectors. 13C + ions are measured as an ion current in a Faraday cup following the fragment analysis magnet.
Importantly AMS counts 14C as individual nuclei and is independent of decay. Around 10% of the 14C in a sample is counted which is 1000-times more efficient than decay counting. Sensitivities approaching 14C /C of ~ 2 x 10-15 or 10 attomoles of 14C can be achieved in mg sized samples.